|
Coproporphyrinogen III
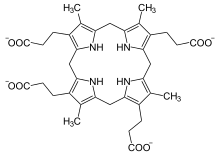
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid. The compound is a porphyrinogen, a class of compounds characterized by a hexahydroporphine core with various side chains. The coproporphyrinogens have the outermost hydrogen atoms of the core replaced by four methyl groups −CH3 (M) and four propionic acid groups −CH2−CH2−COOH (P). In coproporphyrogen III, the order around the outer ring is MP-MP-MP-PM. For comparison, coproporphyrinogen I has them in the sequence MP-MP-MP-MP. heme. Biosynthesis and metabolismIn the main porphyrin biosynthesis pathway, coproporphyrinogen III is derived from uroporphyrinogen III by the action of the enzyme uroporphyrinogen III decarboxylase:  The conversion entails four decarboxylations, which turn the four acetic acid groups −CH2−COOH into methyl groups −CH3, with release of four carbon dioxide molecules.[1][2] Coproporphyrinogen III is further used as a substrate for the enzyme coproporphyrinogen III oxidase which oxidizes and further decarboxylates it to protoporphyrinogen IX. References
|
||||||||||||||||||||||||||||||||