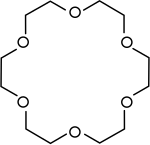
Ether
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Messier 59Messier 59 diambil oleh Hubble.[1]Data pengamatan (J2000 epos)Rasi bintangVirgo[2]Asensio rekta 12j 42m 02.3d[3]Deklinasi +11° 38′ 49″[3]Pergeseran merah410 ± 6 km/s[3]Jarak60 ± 5 Mly (18.3 ± 1.7 Mpc)[4]Magnitudo semu (V)10.6[3]Ciri-ciriJenisE5[3]Ukuran semu (V)5′.4 × 3′.7[3]Penamaan lainNGC 4621,[3] UGC 7858, PGC 42628,[3] CGCG 70-223,[3]…

Minyak nabati adalah Trigliserida yang diekstrak dari tanamans. Beberapa minyak tersebut telah menjadi bagian dari kebudayaan manusia dalam waktu yang lama.[1] Daftar minyak Lihat pula: Minyak goreng Minyak utama Semuanya juga dapat digunakan sebagai minyak bakar. Minyak kelapa, sebuah minyak goreng[2] Minyak jagung, salah satu minyak penting yang dijual sebagai minyak salad dan minyak goreng.[3] Minyak biji kapas, digunakan sebagai minyak salad dan minyak goreng, baik se…

Stasiun Komagawa高麗川駅Pintu masuk stasiun pada September 2020Lokasi336-2 Harajuku, Hidaka-shi, Saitama-ken 350-1205JepangOperator JR EastJalur ■ Jalur Hachikō ■ Jalur Kawagoe Jumlah peron1 peron samping + 1 peron pulauJumlah jalur3LayananPemberhentian busSejarahDibuka15 April 1933 (1933-04-15)Elektrifikasi16 Maret 1996 (1996-03-16)PenumpangFY20154.618 per hari Lokasi pada petaStasiun KomagawaLokasi di JepangSunting kotak info • L • BBantuan penggunaan templat i…

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (يوليو 2019) هذه المقالة تحتاج للمزيد من الوصلات للمقالات الأخرى للمساعدة في ترابط مقالات الموسوعة. فضلًا ساعد في تحسين …

1982 video game 1982 video gameGalactic GladiatorsPublisher(s)Strategic SimulationsPlatform(s)Apple II, IBM PCRelease1982Genre(s)Computer wargame Galactic Gladiators is a 1982 computer wargame published by Strategic Simulations for the Apple II and IBM PC. Gameplay Galactic Gladiators is a game in which the player fights against an alien opponent.[1] Reception David Long reviewed the game for Computer Gaming World, and stated that Easy to learn and fast to play, GG is a great starter gam…

Aerial-warfare branch of the German military during World War II This article is about the air force of Germany during the Second World War. For the current air force of Germany, see German Air Force. For the First World War army-affiliated air force of Germany, see Luftstreitkräfte. For other uses, see Luftwaffe (disambiguation). LuftwaffeEmblem of the Luftwaffe in silverFounded1935Disbanded1946[N 1]Country GermanyAllegiance Adolf HitlerTypeAir forceRoleAerial warfareSizeAircraft:…

DoroKecamatanPeta lokasi Kecamatan DoroNegara IndonesiaProvinsiJawa TengahKabupatenPekalonganPemerintahan • CamatYohantoPopulasi • Total37,656 jiwa (BPS 2.013) jiwaKode Kemendagri33.26.06 Kode BPS3326060 Luas68,45 km²Desa/kelurahan14 Doro (Jawa: ꦢꦫ, translit. Dara) adalah sebuah kecamatan di Kabupaten Pekalongan, Provinsi Jawa Tengah, Indonesia. Kecamatan ini berjarak sekitar 15 Km dari ibu kota Kabupaten Pekalongan ke arah timur. Pusat pemerintahannya …

1963 novel by Kurt Vonnegut This article is about the Kurt Vonnegut novel. For the string figure, see Cat's cradle. For other uses, see Cat's cradle (disambiguation). Cat's Cradle First edition hardback coverAuthorKurt VonnegutOriginal titleCat's CradleCountryUnited StatesLanguageEnglishGenreSatire, science fictionPublisherHolt, Rinehart and WinstonPublication date1963Media typePrint (hardcover and paperback)Pages304ISBN0-385-33348-XOCLC40067116Preceded byMother Night Followe…

Szegi Place in Borsod-Abaúj-Zemplén, HungarySzegiCountry HungaryCountyBorsod-Abaúj-ZemplénArea • Total9.05 km2 (3.49 sq mi)Population (2004) • Total333 • Density36.79/km2 (95.3/sq mi)Time zoneUTC+1 (CET) • Summer (DST)UTC+2 (CEST)Postal code3918Area code47 Szegi is a village in Borsod-Abaúj-Zemplén county, Hungary. External links Street map (in Hungarian) vteBorsod-Abaúj-Zemplén CountyCity with county rights Mi…

Pour les articles homonymes, voir Alpaga (homonymie). Laine d'alpaga. La fibre d'alpaga est la fibre textile donnée par l'alpaga Vicugna pacos. Le terme désigne indifféremment la laine et le tissu produit avec les poils de cet animal. La laine d'alpaga est une fibre de très haut de gamme, plus douce, plus chaude, plus résistante et plus légère que la laine de mouton. On peut tondre l'animal tous les ans, mais sa toison peut être gardée deux, voire trois ans. Les poils mesurent de 15 à …

Ostrakon abad ke-19 yang terinskripsi dengan bagian dari Sindiran Perdagangan. Torino, Museo Egizio Sindiran Perdagangan, juga disebut Instruksi Dua-Kheti, adalah sebuah karya sastra Mesir kuno didaktik.[1] Karya tersebut mengambil bentuk sebuah Instruksi, yang terdiri dari sebuah naskah dari Sile yang bernama Dua-Kheti kepada putranya Pepi. Pengarangnya dianggap oleh beberapa orang juga mengkomposisikan Instruksi Amenemhat.[2] Penggunaan dalam Ben Sira Kisah Mesir tersebut diruj…

Brazilian rock/blues band This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: O Bando do Velho Jack – news · newspapers · books · scholar · JSTOR (August 2009) (Learn how and when to remove this message) O Bando do Velho JackBackground informationOriginCampo Grande, MS, BrazilGenresBlues, classic rock and southern …

Person who makes announcements in an audio medium or a physical location This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Announcer – news · newspapers · books · sch…

1980 film by Jeannot Szwarc Somewhere in TimeTheatrical film posterDirected byJeannot SzwarcScreenplay byRichard MathesonBased onBid Time Returnby Richard MathesonProduced by Stephen Deutsch Ray Stark (uncredited) Starring Christopher Reeve Jane Seymour Christopher Plummer Teresa Wright CinematographyIsidore MankofskyEdited byJeff GoursonMusic byJohn BarryProductioncompanyRastarDistributed byUniversal PicturesRelease date October 3, 1980 (1980-10-03) Running time103 minutesCountry…

Questa voce o sezione sull'argomento attori italiani non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. Il Mago Forest nel 2022 Mago Forest o Mr. Forest, pseudonimo di Michele Foresta (Nicosia, 22 febbraio 1961), è un comico, showman e conduttore televisivo italiano. Indice 1 Biografia 2 Vita privata 3 Filmogra…

Sceaux 行政国 フランス地域圏 (Région) イル=ド=フランス地域圏県 (département) オー=ド=セーヌ県郡 (arrondissement) アントニー郡小郡 (canton) 小郡庁所在地INSEEコード 92071郵便番号 92330市長(任期) フィリップ・ローラン(2008年-2014年)自治体間連合 (fr) メトロポール・デュ・グラン・パリ人口動態人口 19,679人(2007年)人口密度 5466人/km2住民の呼称 Scéens地理座標 北緯48度46�…

Mosque in Cairo, Egypt This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources.Find sources: Mosque of Khushqadam el-Ahmadi – news · newspapers · books · scholar · JSTOR (February 2012) The Mosque of Khushqadam el-Ahmadi is on el-Seyufia Street in Cairo and was built in 1366. The building was originally the palace of Emir …

Yisrael BarzilaiLahir1 Oktober 1913Tempat lahirNieszawa, Kekaisaran RusiaTahun aliyah1934Meninggal dunia12 Juni 1970Knesset3, 4, 5Faksi yang diwakili di Knesset1955–1965MapamJabatan menteri1955–1961Menteri Kesehatan1958–1959Menteri Layanan Pos1966–1969Menteri Kesehatan1969–1970Menteri tanpa Portofolio Yisrael Barzilai (Ibrani: ישראל ברזילי, nama lahir Yisrael Eisenberg 1 Oktober 1913 – 12 Juni 1970) adalah seorang politikus Israel yang menjabat sebagai menter…

Extratropical cyclone This article or section appears to contradict the article Tornadoes of 2010 on number of tornadoes in the event (this article claims 69, but that article claims 87). Please see the talk page for more information. (July 2021) October 2010 North American storm complexSatellite image of the storm complex at peak intensity, on October 27, 2010. TypeExtratropical cyclone, Blizzard, Derecho, Tornado outbreak, WindstormFormedOctober 23, 2010DissipatedNovember 5, 2010 Lowest p…

European balance of power in the 19th century This article is about the 19th-century diplomatic term. For the jazz album, see European Concert. Concert of Europe1815 to 1848/1860s – 1871 to 1914The national boundaries within Europe as set by the Congress of Vienna, 1815Including Regency era Bourbon Restoration Revolutions of 1830 Revolutions of 1848 Causes of World War I Chronology Napoleonic era League of Nations The Concert of Europe was a general agreement among the great powers of…