|
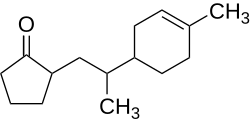
2-(2-(4-Methyl-3-cyclohexen-1-yl)propyl)cyclopentanone
2-[2-(4-Methyl-3-cyclohexen-1-yl)propyl]cyclopentanone (trade name by Givaudan: Nectaryl) is an organic compound belonging to the group of ketones and cycloalkanes. The compound is used as a fragrance. SynthesisThe synthesis of the compound is carried out by a radical addition of cyclopentanone to (+)-limonene under oxygen in acetic acid. As a catalyst, manganese(II) acetate and cobalt(II) acetate are used.[2] PropertiesThe flash point of the compound is 162.5 °C, and the autoignition temperature is 294 °C.[1] The specific rotation is reported to be [α]D20=+228–235° (1 M; chloroform)[2] In general, the compound features a fruity apricot-like odor. Of the four stereo isomers, (2R,2′S,1′′R)-Nectaryl and (2R,2′R,1′′R)-Nectaryl contribute especially to the compound's odor, the odor detection threshold lies at 0.094 ng·l−1 and 0.112 ng·l−1, respectively. In contrast to that, the other stereo isomers show an unspecific fruity odor, the odor detection threshold are 11.2 ng·l−1 and 14.9 ng·l−1 which is much higher.[2][3] The tenacity on blotter, the time during which the compound is smellable with unchanged characteristics,[4]: 51 is reported to be three weeks.[4]: 52 UsesThe substance is used as a fragrance in exemplary air conditioning products, perfumes and polishes.[5] Literature
Information related to 2-(2-(4-Methyl-3-cyclohexen-1-yl)propyl)cyclopentanone |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||