Dichloro(1,3-bis(diphenylphosphino)propane)nickel
Dichloro[1,3-bis(diphenylphosphino)propane]nickel
Names
Systematic IUPAC name
Dichloro[1,3-propanediylbis(diphenylphosphanuide-κP)]nickel
Other names
1,3-bis(diphenylphosphino)propanenickel(II) chloride;
NiCl2 (dppp)
Identifiers
ChemSpider
ECHA InfoCard 100.132.628
EC Number
InChI=1S/C27H26P2.2Cl.Ni/c1-5-14-24(15-6-1)28(25-16-7-2-8-17-25)22-13-23-29(26-18-9-3-10-19-26)27-20-11-4-12-21-27;;;/h1-12,14-21H,13,22-23H2;;;
Cl[Ni]1([P](c2ccccc2)(c3ccccc3)CCC[P]1(c4ccccc4)c5ccccc5)Cl
Properties
C 27 H 26 Cl 2 Ni P 2
Molar mass
−1
Appearance
Orange to red-orange powder
Melting point
213 °C (415 °F; 486 K)
Insoluble
Hazards
GHS labelling
[ 1]
Danger [ 1]
H315 , H317 , H319 , H334 , H335 , H350 [ 1]
P201 , P261 , P280 , P305+P351+P338 , P308+P313 [ 1]
Safety data sheet (SDS)
External SDS
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Dichloro[1,3-bis(diphenylphosphino)propane]nickel a coordination complex with the formula NiCl2 (dppp); where dppp is the diphosphine 1,3-bis(diphenylphosphino)propane . It is used as a catalyst in organic synthesis. The compound is a bright orange-red crystalline powder.
Structure and properties
While the electronic and solid-state structure of the chloride congener is not known (due to low solubility in common analytical solvents), several studies have been carried out on the bromo and iodo derivatives.[ 2] dichloro(1,2-bis(diphenylphosphino)ethane)nickel adopts a static square-planar (diamagnetic) structure in solution.
Preparation
NiCl2 (dppp) is prepared by combining equal molar portions of nickel(II) chloride hexahydrate with 1,3-bis(diphenylphosphino)propane in 2-propanol.[ 3]
Ni(H2 O)6 Cl2 + dppp → NiCl2 (dppp) + 6 H2 O
Reactions
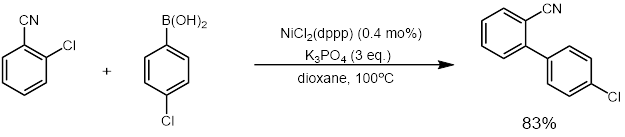
NiCl2 (dppp) in an effective catalyst for coupling reactions such as the Kumada coupling [ 3] Suzuki reactions (example below).[ 4] enol ethers , dithioacetals , and vinyl sulfides to olefins .[ 5] [ 6]
References
^ a b c d "1,3-Bis(diphenylphosphino)propane Nickel(II) Chloride" . American Elements . Retrieved September 6, 2018 .^ Van Hecke, Gerald R.; Horrocks, Jr., William DeW. (1966). "Ditertiary Phosphine Complexes of Nickel. Spectral, Magnetic, and Proton Resonance Studies. A Planar-Tetrahedral Equilibrium". Inorganic Chemistry . 5 (11): 1968– 1974. doi :10.1021/ic50045a029 . ^ a b Kumada, Makota; Tamao, Kohei; Sumitani, Koji (1978). "Phosphine-Nickel Complex Catalyzed Cross-Coupling of Grignard Reagents with Aryl and Alkenyl Halides: 1,2-Dibutylbenzene". Org. Synth . 58 : 127. doi :10.15227/orgsyn.058.0127 . ^ Zhao, Yu-Long; Li, You; Li, Shui-Ming; Zhou, Yi-Guo; Sun, Feng-Yi; Gao, Lian-Xun; Han, Fu-She (1 June 2011). "A Highly Practical and Reliable Nickel Catalyst for Suzuki-Miyaura Coupling of Aryl Halides". Advanced Synthesis & Catalysis . 353 (9): 1543– 1550. doi :10.1002/adsc.201100101 . ^ Tien-Yau Luh; Tien-Min Yuan. "Cross-Coupling Reactions". Encyclopedia of Reagents for Organic Synthesis doi :10.1002/047084289X.rd100.pub2 .
^ Ljungdahl, Thomas; Bennur, Timmanna; Dallas, Andrea; Emtenaes, Hans; Maartensson, Jerker (2008). "Two Competing Mechanisms for the Copper-Free Sonogashira Cross-Coupling Reaction". Organometallics 27 (11): 2490– 2498. doi :10.1021/om800251s .
Information related to Dichloro(1,3-bis(diphenylphosphino)propane)nickel