This article is about 1,3-dioxolane. For the other dioxolane isomer, see
1,2-dioxolane .
Dioxolane[ 1]
Names
Preferred IUPAC name
Systematic IUPAC name
Other names
Dioxolane
5-Crown-2
Formal glycol
[ 2]
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.010.422
EC Number
UNII
UN number
1166
InChI=1S/C3H6O2/c1-2-4-5-3-1/h1-3H2
Y Key: SNQXJPARXFUULZ-UHFFFAOYSA-N
Y InChI=1/C3H6O2/c1-2-4-5-3-1/h1-3H2
Key: SNQXJPARXFUULZ-UHFFFAOYAS
Properties
C3 H6 O2
Molar mass
74.08 g/mol
Density
1.06 g/cm3
Melting point
−95 °C (−139 °F; 178 K)
Boiling point
75 °C (167 °F; 348 K)
Hazards
GHS labelling[ 4]
Danger
H225
P210 , P233 , P240 , P241 , P242 , P243 , P280 , P303+P361+P353 , P370+P378 , P403+P235 , P501
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
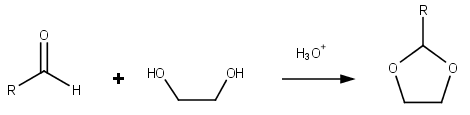
Dioxolane is a heterocyclic acetal with the chemical formula (CH2 )2 O2 CH2 . It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2 ) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4 O2 rings are called dioxanes . The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide . 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals .
As a class of compounds
Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol .[ 5]
synthesis of dioxolane group (+)-cis -Dioxolane is the trivial name for L -(+)-cis -2-methyl-4-trimethylammoniummethyl-1,3-dioxolane iodide which is a muscarinic acetylcholine receptor agonist .
Protecting groups
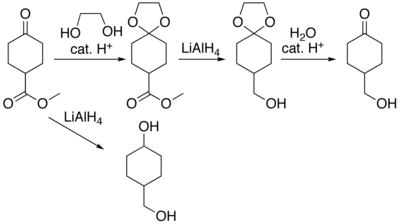
Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls[ 6] [ 7] lithium aluminium hydride reduction will produce 4-hydroxymethylcyclohexanol. The ester functional group can be reduced without affecting the ketone by protecting the ketone as a ketal . The ketal is produced by acid catalysed reaction with ethylene glycol , the reduction reaction carried out, and the protecting group removed by hydrolysis to produce 4-hydroxymethylcyclohexanone.
NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds.[ 6] [ 7] benzaldehyde can be achieved in water in five minutes at 30 °C.[ 8]
PhCH(OCH2 )2 + H2 O
→
30 °C / 5 min
NaBAr
4
{\displaystyle {\ce {->[{\ce {NaBAr4}}][{\text{30 °C / 5 min}}]}}}
HOCH2 CH2 OH
Natural products
Neosporol is a natural product that includes a 1,3-dioxolane moiety , and is an isomer of sporol which has a 1,3-dioxane ring.[ 9] total synthesis of both compounds has been reported, and each includes a step in which a dioxolane system is formed using trifluoroperacetic acid (TFPAA), prepared by the hydrogen peroxide – urea method.[ 10] [ 11] anhydrous peracid,[ 12] side reactions .[ 10]
CF3 3 H2 2 2 2 CF3 CF3 CO(NH2 2 In the case of neosporol, a Prilezhaev reaction [ 13] allyl alcohol precursor to an epoxide , which then undergoes a ring-expansion reaction with a proximate carbonyl functional group to form the dioxolane ring.[ 10] [ 11]
A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.[ 9]
See also
References
^ 1,3-Dioxolane at Sigma-Aldrich ^ formal glycol - PubChem Public Chemical Database ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) . Cambridge: The Royal Society of Chemistry . 2014. p. 145. doi :10.1039/9781849733069-FP001 . ISBN 978-0-85404-182-4 ^ "1,3-Dioxolane" . pubchem.ncbi.nlm.nih.gov .^ R. A. Daignault, E. L. Eliel (1973). "2-Cyclohexyloxyethanol (involves acetalisation of cyclohexanone)" . Organic Syntheses Collected Volumes , vol. 5, p. 303^ a b Greene, Theodora W.; Wuts, Peter G. M. (1999). "Dimethyl acetals". Greene's Protective Groups in Organic Synthesis Wiley-Interscience . pp. 297– 304, 724– 727. ISBN 9780471160199 Archived from the original on December 3, 2016. Retrieved June 20, 2017 . ^ a b Greene, Theodora W.; Wuts, Peter G. M. (1999). "1,3-Dioxanes, 1,3-Dioxolanes". Greene's Protective Groups in Organic Synthesis Wiley-Interscience . pp. 308– 322, 724– 727. ISBN 9780471160199 Archived from the original on December 7, 2016. Retrieved June 20, 2017 . ^ Chang, Chih-Ching; Liao, Bei-Sih; Liu, Shiuh-Tzung (2007). "Deprotection of Acetals and Ketals in a Colloidal Suspension Generated by Sodium Tetrakis(3,5-trifluoromethylphenyl)borate in Water". Synlett 2007 (2): 283– 287. doi :10.1055/s-2007-968009 . ^ a b Pirrung, Michael C.; Morehead, Andrew T.; Young, Bruce G., eds. (2000). "10. Neosporol, Sporol" . Part B: Bicyclic and Tricyclic Sesquiterpenes . The Total Synthesis of Natural Products. Vol. 11. John Wiley & Sons . pp. 222– 224. ISBN 9780470129630 ^ a b c Ziegler, Fredrick E.; Metcalf, Chester A.; Nangia, Ashwini; Schulte, Gayle (1993). "Structure and total synthesis of sporol and neosporol". J. Am. Chem. Soc. 115 (7): 2581– 2589. doi :10.1021/ja00060a006 . ^ a b Caster, Kenneth C.; Rao, A. Somasekar; Mohan, H. Rama; McGrath, Nicholas A.; Brichacek, Matthew (2012). "Trifluoroperacetic Acid". e-EROS Encyclopedia of Reagents for Organic Synthesis doi :10.1002/047084289X.rt254.pub2 . ISBN 978-0471936237 ^ Cooper, Mark S.; Heaney, Harry ; Newbold, Amanda J.; Sanderson, William R. (1990). "Oxidation Reactions Using Urea–Hydrogen Peroxide; A Safe Alternative to Anhydrous Hydrogen Peroxide". Synlett 1990 (9): 533– 535. doi :10.1055/s-1990-21156 . ^ Hagen, Timothy J. (2007). "Prilezhaev reaction" . In Li, Jie Jack; Corey, E. J. (eds.). Name Reactions of Functional Group Transformations . John Wiley & Sons . pp. 274– 281. ISBN 9780470176504
External links
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (and prodrugs )
Information related to Dioxolane




![{\displaystyle {\ce {->[{\ce {NaBAr4}}][{\text{30 °C / 5 min}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3fb42849d6133fe653a7fe5dd019b12e0f6184b5)